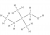
It is 14 hydrogens (D).
The rationale and a good approach for working it out:
Butane, with 4 carbons would have 2n + 2 = 2x4 + 2 = 10 hydrogens
Butanol, with substitution of a hydrogen (H) attached to C1 for a hydroxyl (-OH) group doesn’t change the number of hydrogens from the base alkanes.
With substitution of each of both hydrogens (H) attached to C2, for a methyl (CH3) group, each substitution increases the number of hydrogens by 3-1 = 2, with two substitutions increasing the total number by 2x2 = 4.
Hence, total number of hydrogens is 10+4 = 14.