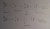b) Brief summary of how radioisotopes are produced:
Two main ways required for HSC Chemistry:
- Nuclear Reactor (such as ones in Lucas Heights)
- used for creating neutron-rich isotopes by neutron (
neutral) bombardment
- Particle Accelerators (such as cyclotrons, linear particle accelerators)
- used for creating neutron-deficient isotopes by bombardment by
charged particles (such as protons, which have a positive charge)
- in order for charged particles to be incorporated into elements, they must be "accelerated" in a suitable manner so that they can overcome the electrostatic repulsions brought about by the charged particles (eg. protons) already in that element
Now, to answer your question:
Carbon-11 is made from Boron-10. Now, the atomic number (i.e. number of protons) of Boron-10 is 5, and the atomic number of Carbon-11 is 6 (one more proton that Boron-10).
Remember the atomic number of an element is what makes it unique and allows us to identify what element it is.
So, to get Carbon-11 from Boron-10, we have to add an extra proton to Boron-10.
Now, protons are positively charged. And from above, we know that charged particles such as protons need to be accelerated into Boron-11 in order for it to overcome the electrostatic repulsions caused by the protons already in Boron-10.
So therefore, a particle accelerator, such as a cyclotron, and not a nuclear reactor, should be used to create Carbon-11 from Boron-10.
c)According to the zone/belt of stability used for radioisotopes, isotopes with atomic number less than 20 (such as Carbon-11, which has an atomic number of 6), should have a neutron: proton ratio of 1:1 in order for it to be stable.
View attachment 31727
However, Carbon-11 has 6 protons, and 5 neutrons {remember: the "11" in Carbon-11 is the atomic mass, which means protons PLUS neutrons, so to get the number of neutrons given that there are 6 protons in Carbon-11, just subtract 6 from 11: 11 - 6 = 5).
This gives a neutron: proton ratio of 5:6, which is not a ratio of 1:1. Therefore, Carbon-11 must radioactively decay in order to achieve a stable neutron: proton ratio.
Again, from b), we know that particle accelerators produce neutron deficient isotopes, so that means Carbon-11 is neutron deficient. Neutron deficient isotopes tend to decay through electron capture or positron emission (positrons are electrons with a positive charge) (as seen through the graph above) in order to achieve more stable neutron: proton ratio. Gamma rays may be emitted as well.
Thus, we come up with the following equations (which must be included in your answer to this question):
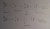
{NOTE: make sure to balance protons/neutrons on either side of the equation}
And since Carbon-11 can emit gamma rays when it decays into Boron-11, gamma cameras may be used to scan the body.
I hope this helps! Good luck with your studies!