-
Looking for HSC notes and resources? Check out our Notes & Resources page
?? (1 Viewer)
- Thread starter YourLocalDumbAss
- Start date
Life'sHard
Well-Known Member
- Joined
- May 24, 2021
- Messages
- 1,102
- Gender
- Male
- HSC
- 2021
- Uni Grad
- 2025
YourLocalDumbAss
Well-Known Member
Seems like more of a biology content than a Chemistry oneView attachment 32933
Something like this.
Life'sHard
Well-Known Member
- Joined
- May 24, 2021
- Messages
- 1,102
- Gender
- Male
- HSC
- 2021
- Uni Grad
- 2025
Nah this is chem and it should have shown up in the textbook.Seems like more of a biology content than a Chemistry one
YourLocalDumbAss
Well-Known Member
I must of missed it, thanks thoNah this is chem and it should have shown up in the textbook.
5uckerberg
Well-Known Member
- Joined
- Oct 15, 2021
- Messages
- 562
- Gender
- Male
- HSC
- 2018
Oh, what is this? The resemblance of saponification. I think we can bring up a few ideas like the head of a negative charge is hydrophilic and as such interacts with the positive charges from the outside while the tail of the negative charge is hydrophobic and as such will interact with the grease and then splits up the grease from dirty dishes. I am just throwing stuff up from the old syllabus. If someone better than me could answer that question I will be glad.
The sodium salts of long chain fatty acids consist of two parts: a non-polar hydrophobic ‘tail’ consisting of fatty acids; and a polar, hydrophilic, charged ‘head’ consisting of the sodium salt of the alkanoic acid, as shown below.

A micelle forms when sodium salts assemble so that the long hydrophobic tails all point inwards and the polar heads all sit on the outside of the micelle.
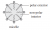
The hydrophobic tails embed themselves in the grease. The hydrophilic heads are attracted to the water and lift the grease off the dirty dishes to reform a micelle that then remains suspended in water.

An important thing to consider is that while the question requires a diagram of only a single micelle, diagrams of micelle formation or action such as those above may help to develop high-quality responses.
Regarding the syllabus, as Life'sHard mentioned, this is indeed part of the syllabus, Module 7: Organic Chemistry to be precise.
I hope this helps!

A micelle forms when sodium salts assemble so that the long hydrophobic tails all point inwards and the polar heads all sit on the outside of the micelle.

The hydrophobic tails embed themselves in the grease. The hydrophilic heads are attracted to the water and lift the grease off the dirty dishes to reform a micelle that then remains suspended in water.

An important thing to consider is that while the question requires a diagram of only a single micelle, diagrams of micelle formation or action such as those above may help to develop high-quality responses.
Regarding the syllabus, as Life'sHard mentioned, this is indeed part of the syllabus, Module 7: Organic Chemistry to be precise.
I hope this helps!
YourLocalDumbAss
Well-Known Member
Thank you Jimmy, now got to read more throughly next time without missing these key information/concepts. 

Life'sHard
Well-Known Member
- Joined
- May 24, 2021
- Messages
- 1,102
- Gender
- Male
- HSC
- 2021
- Uni Grad
- 2025
Jimmy jimmy jimmy. Where do you keep finding these solutions? What's your secret lol?The sodium salts of long chain fatty acids consist of two parts: a non-polar hydrophobic ‘tail’ consisting of fatty acids; and a polar, hydrophilic, charged ‘head’ consisting of the sodium salt of the alkanoic acid, as shown below.
View attachment 32934
A micelle forms when sodium salts assemble so that the long hydrophobic tails all point inwards and the polar heads all sit on the outside of the micelle.
View attachment 32935
The hydrophobic tails embed themselves in the grease. The hydrophilic heads are attracted to the water and lift the grease off the dirty dishes to reform a micelle that then remains suspended in water.
View attachment 32936
An important thing to consider is that while the question requires a diagram of only a single micelle, diagrams of micelle formation or action such as those above may help to develop high-quality responses.
Regarding the syllabus, as Life'sHard mentioned, this is indeed part of the syllabus, Module 7: Organic Chemistry to be precise.
I hope this helps!
specificagent1
Well-Known Member
- Joined
- Aug 24, 2021
- Messages
- 1,969
- Gender
- Male
- HSC
- 2021
@YourLocalDumbAss i'm very confused. It says HSC 2024 and you were just asking about investigating science but now you're doing year 12 chem?
YourLocalDumbAss
Well-Known Member
I share this account with an older sibling. As much of you must be thinking that we should make separate account, I can’t be bothered making a whole new account and starting from scratch, so I remain on this account with my older sister till she finishes the HSC.@YourLocalDumbAss i'm very confused. It says HSC 2024 and you were just asking about investigating science but now you're doing year 12 chem?
YourLocalDumbAss
Well-Known Member
Jimmy must of found the PEM 2020 Chemistry Solutions, which I don’t have.Jimmy jimmy jimmy. Where do you keep finding these solutions? What's your secret lol?
Sorry for the leak of information Jimmy.
Life'sHard
Well-Known Member
- Joined
- May 24, 2021
- Messages
- 1,102
- Gender
- Male
- HSC
- 2021
- Uni Grad
- 2025
I didn’t even realised you put it in the original post lmao. But still for other posts Jimmy always nails finding the solutions.Jimmy must of found the PEM 2020 Chemistry Solutions, which I don’t have.
Sorry for the leak of information Jimmy.
I do not have access to PEM papers actually haha.
Please be careful not to engage in promoting/sharing copyrighted material guys. Thanks! 
specificagent1
Well-Known Member
- Joined
- Aug 24, 2021
- Messages
- 1,969
- Gender
- Male
- HSC
- 2021
does copy right apply to educational use?Please be careful not to engage in promoting/sharing copyrighted material guys. Thanks!
DheerChoudhury
Active Member
- Joined
- Nov 1, 2019
- Messages
- 119
- Gender
- Male
- HSC
- 2021
ohhhhh my bad lolPlease be careful not to engage in promoting/sharing copyrighted material guys. Thanks!
While the use of trial HSC exam papers is indeed for educational purposes, papers that are protected by copyright (including CSSA, Independent and others, such as PEM) cannot be shared on here for 2 reasons:does copy right apply to educational use?Any lawyers here on BOS?
- It is against the rules of this forum.
- Such papers must be purhcased by schools/individuals that wish to use them, unlike those produced by individual schools, which can be freely shared/accessed.
YourLocalDumbAss
Well-Known Member
That’s why I’m seeing resources that get deleted when uploaded, but lucky I download them before the copyright strikes and the resource is deleted. Thanks for the information Jimmy.While the use of trial HSC exam papers is indeed for educational purposes, papers that are protected by copyright (including CSSA, Independent and others, such as PEM) cannot be shared on here for 2 reasons:
This also means that you are not to request and/or share copyrighted material (this also applies to textbooks). However, you are allowed to have discussions about the papers/textbooks.
- It is against the rules of this forum.
- Such papers must be purhcased by schools/individuals that wish to use them, unlike those produced by individual schools, which can be freely shared/accessed.

No worries. Going forward, you are encouraged to report such material to us so that we can take appropriate action.That’s why I’m seeing resources that get deleted when uploaded, but lucky I download them before the copyright strikes and the resource is deleted. Thanks for the information Jimmy.


