wait how many ppl roughly attend peak chem then for yr 11..they usually post the top 50 rankings for the cohort to see, it'll probably be done over the christmas break
zhangs or ace for CHEMISTRY? (1 Viewer)
- Thread starter enip
- Start date
asiansubjects
New Member
- Joined
- Apr 22, 2020
- Messages
- 14
- Gender
- Male
- HSC
- 2020
they posted it on their discord server beforeohhh where do they post it?
asiansubjects
New Member
- Joined
- Apr 22, 2020
- Messages
- 14
- Gender
- Male
- HSC
- 2020
not too sure on the numbers myself but they have quite a lot of classeswait how many ppl roughly attend peak chem then for yr 11..
not anyone that i know of, and plus the last question i doubt someone got it but maybe who knows?do you know if anyone got 50? i havent heard of anyone getting it i think 49 might be highest
eternallyboreduser
Well-Known Member
- Joined
- Jul 4, 2023
- Messages
- 548
- Gender
- Undisclosed
- HSC
- N/A
bruh legit and misreading the q too, although i can accept this one circlinf thr wrong bubble is depressing. imma start circling on the actual sheet first cos usually i circle it on the paper and then copy it down onto the mcq sheetdang but still a high mark. bruh circling the wrong bubble is just sad
eternallyboreduser
Well-Known Member
- Joined
- Jul 4, 2023
- Messages
- 548
- Gender
- Undisclosed
- HSC
- N/A
hmmm yeah i go to the canley campus and highest in my class was 47.5not anyone that i know of, and plus the last question i doubt someone got it but maybe who knows?
eternallyboreduser
Well-Known Member
- Joined
- Jul 4, 2023
- Messages
- 548
- Gender
- Undisclosed
- HSC
- N/A
apparently theres like around 200 in physics so there should be more for chem since its more popular at peakwait how many ppl roughly attend peak chem then for yr 11..
eternallyboreduser
Well-Known Member
- Joined
- Jul 4, 2023
- Messages
- 548
- Gender
- Undisclosed
- HSC
- N/A
my friend does physixs and peak and he said he was ranked like 20 smth outta 200 smthapparently theres like around 200 in physics so there should be more for chem since its more popular at peak
oh wow that is a large cohort. chem is gonna be hugeeeeeeeeapparently theres like around 200 in physics so there should be more for chem since its more popular at peak
which q did u misreadbruh legit and misreading the q too, although i can accept this one circlinf thr wrong bubble is depressing. imma start circling on the actual sheet first cos usually i circle it on the paper and then copy it down onto the mcq sheet
eternallyboreduser
Well-Known Member
- Joined
- Jul 4, 2023
- Messages
- 548
- Gender
- Undisclosed
- HSC
- N/A
uhhh i dont even remember i think i lost the exam paperwhich q did u misread
eternallyboreduser
Well-Known Member
- Joined
- Jul 4, 2023
- Messages
- 548
- Gender
- Undisclosed
- HSC
- N/A
i remember that it was only worth a mark tho and im pre sure it was an easy q toouhhh i dont even remember i think i lost the exam paper
eternallyboreduser
Well-Known Member
- Joined
- Jul 4, 2023
- Messages
- 548
- Gender
- Undisclosed
- HSC
- N/A
im very curious about my rank though
yeah the average was around 80-85 so you chilling. also i heard that if top. 50, then we get a bonus/discount but not 100% certai. have u heard ???im very curious about my rank though
eternallyboreduser
Well-Known Member
- Joined
- Jul 4, 2023
- Messages
- 548
- Gender
- Undisclosed
- HSC
- N/A
damnn rlly? That would be sick if it’s true but ive never heard of thatyeah the average was around 80-85 so you chilling. also i heard that if top. 50, then we get a bonus/discount but not 100% certai. have u heard ???
Last edited:
eternallyboreduser
Well-Known Member
- Joined
- Jul 4, 2023
- Messages
- 548
- Gender
- Undisclosed
- HSC
- N/A
My class average was like in the high 70’s i think lolyeah the average was around 80-85 so you chilling. also i heard that if top. 50, then we get a bonus/discount but not 100% certai. have u heard ???
don't have the paper to show but question was like why is cyclopropane more reactive than propane (with diagram)Care to share any of the difficult questions?
That's a good question, though not one that really fits inside the HSC syllabus.don't have the paper to show but question was like why is cyclopropane more reactive than propane (with diagram)
Ring strain is a destabilising factor for ring systems except when they have six atoms in the ring - Wikipedia has an article about it - and the problem with bond angles away from the ideal tetrahedral angle is intuitively obvious. The contribution from eclipsed rather than staggered conformations is also not difficult to appreciate in a qualitative sense, though the syllabus does not really go into those areas either.
The effect can be seen in its reactivity, for example. Cyclopropane reacts with HBr to yield 1-bromopropane, an addition reaction that is akin to the behaviour of propene but not propane.
Quantifying the effect of ring strain is well beyond the syllabus, though quantified effects can be seen in the elevation of the enthalpies of combustion. Estimating the enthalpy of combustion from bond energies for cyclopropane (in gas phase) gives:
Cyclopropane: C3H6 (g) + 4.5 O2 (g) ---> 3 CO2 (g) + 3 H2O (g)
} + 6 \times \text{BE(C-H)} + 4.5 \times \text{BE(O=O)} - \left[6 \times \text{BE(C=O)} + 6 \times \text{BE(H-O)}\right] \\ &= 3 \times 348 + 6 \times 413 + 4.5 \times 495 - \left[6 \times 799 + 6 \times 463\right] \\ &= 5749.5 - 7572 \\ &= -1822.5\ \text{kJ mol}^{-1} \end{align*})
Adjusting back to standard states by using:
H2O (g) ---> H2O (l) 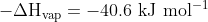
gives an estimate of the enthalpy of combustion as
The actual value is
This 150 kJ mol-1 difference reflects that the three strained carbon-carbon bonds in the cyclopropane ring are weaker than a typical carbon-carbon bond. In fact, assuming that the strain only affects the carbon-carbon bond strength (which isn't true, but the effect on the carbon-hydrogen bond strengths will be significantly smaller in magnitude), we can calculate:
In other words, compared to the typical / average BE(C-C) = +348 kJ mol-1, the carbon-carbon bonds in cyclopropane are only about 75% as strong.
There is the basis for a good, challenging, question that builds on content in modules 1, 4, and 7 (at least).
Inhstwant99.95atar
New Member
Whats’s wrong with peak?hi, I go to PEAK for chemistry (year 11), but I want to change next term. I was thinking either Zhangs or Ace. I also thought matrix but i heard that they were a bit on the easier side (not 100% sure about that tho). Anyways can someone who went to either or them, or just in general suggest which one would suit me. I am looking for an organised tutoring which is more on the harder end, like having harder homework and exams. thanks
I currently attend Year 12 lessons for chemistry there, and I find that the content they teach you is “all you need to know,” places like matrix like to waffle a lot. However, I did see zhang’s state ranks look very appealing, but the theory is very hard to look at (looks like a textbook).
They also like throwing you straight into hard questions, which makes you actually think. No spoon feeding haha.
