-
Looking for HSC notes and resources? Check out our Notes & Resources page
shouldnt h2o be tetrahedral geometry (1 Viewer)
- Thread starter kkk579
- Start date
Average Boreduser
Rising Renewal
no. 2 o-h bonds and 2 lone pair. It's angular bent sir.
Last edited:
kkk579
hello
No but isnt the shape bent and the geometry is tetrahedralno. 2 o-h bonds and a lone pair. It's angular bent sir.
Average Boreduser
Rising Renewal
fkNo but isnt the shape bent and the geometry is tetrahedral
kkk579
hello
yeah thats shape not geometry thoughi dont rememeber yr 11 stuff but doesnt the electron geometry maily depend on the bonded electrons, plus its symmetry so maybe thats why its bent
Susu123
Well-Known Member
- Joined
- Jul 5, 2023
- Messages
- 415
- Gender
- Female
- HSC
- 2024
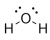 its like this, so all the electron pairs repel each other, so the electron geometry causes the the hydrogens and their shared electrons to also be repelled, leading to the bent shape. like since there are 2 bonds, it can't be tetrahedral cuz that requires more than two bonds idk if that makes sense, there should be a yt video on this
its like this, so all the electron pairs repel each other, so the electron geometry causes the the hydrogens and their shared electrons to also be repelled, leading to the bent shape. like since there are 2 bonds, it can't be tetrahedral cuz that requires more than two bonds idk if that makes sense, there should be a yt video on thiswizzkids
Well-Known Member
- Joined
- Jul 13, 2016
- Messages
- 341
- Gender
- Undisclosed
- HSC
- 1998
You should recall from valence shell electron-pair repulsion theory that the bonding and the non-bonding electron pairs occupy positions as far away from each other as possible. Yes, that means the electron pairs are in a tetrahedral arrangement, but it also means the two hydrogens are in a bent orientation, the bond angle is 104.5o. If it was a proper tetrahedral arrangement the bond angle would be 109o.
kkk579
hello
? ive learnt that geometry of h2o is tetrahedral and the shape is bent at tutor from smo that state ranked chem so idk LOL. but the q asks for geometry so i dont get how its bent.View attachment 43672its like this, so all the electron pairs repel each other, so the electron geometry causes the the hydrogens and their shared electrons to also be repelled, leading to the bent shape. like since there are 2 bonds, it can't be tetrahedral cuz that requires more than two bonds idk if that makes sense, there should be a yt video on this
i think ur right but shape and geometry generally are used interchangeably and this q has the point of making u label different geometries. most often probably molecular geometry rather than electron geometry is assumedNo but isnt the shape bent and the geometry is tetrahedral

