erfanau123
Member
- Joined
- Sep 24, 2011
- Messages
- 58
- Gender
- Male
- HSC
- 2011
fusion of hydrogen atoms in the sun to form a helium nucleus can be represented by the equation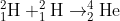
calculate the mass defect of the event and the energy produced
(specimen 4 marks )
calculate the mass defect of the event and the energy produced
(specimen 4 marks )

