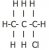
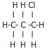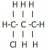Hi guys,
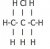
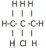
I'm having trouble with drawing the structural formulae of structural isomers. For example, I don't understand why is there only two isomers can be drawn from C3H7Cl (1-cholropropane and 2-chloropropane)? Can't it be 3-chloropropane? Or the following?
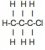
Also, for this question (b), the product should contains hydrogen gas, doesn't it? However I don't think there is any hydrogen atoms among the reactants...

Any help would be appreciated!!
I'm having trouble with drawing the structural formulae of structural isomers. For example, I don't understand why is there only two isomers can be drawn from C3H7Cl (1-cholropropane and 2-chloropropane)? Can't it be 3-chloropropane? Or the following?
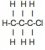
Also, for this question (b), the product should contains hydrogen gas, doesn't it? However I don't think there is any hydrogen atoms among the reactants...

Any help would be appreciated!!