-
Looking for HSC notes and resources? Check out our Notes & Resources page
Why does iron thiocyanate have a colour similar to blood (1 Viewer)
- Thread starter erucibon
- Start date
It is a complexation reaction between the Fe metal ion and the SCN Ligand - assuming you are in yr 11/new year 12, a ligand is usually a non metal ion/molecule which bonds to a central metal cation in a coordinate covalent bond. Thus, because of the way the SCN and Fe interact to form a unique complex, they form a blood red unique colour - not sure if this answers or helps
Also, for each complex formed, they have different and identifiable colours. This can help identify ions in solution. For example, if you are trying to determine the presence of Fe3+ ions in a solution, just add some KSCN and if a blood red colour appears, then the Fe is present
You will learn this in Mod 8, the last module of HSC Chem
Also, for each complex formed, they have different and identifiable colours. This can help identify ions in solution. For example, if you are trying to determine the presence of Fe3+ ions in a solution, just add some KSCN and if a blood red colour appears, then the Fe is present
You will learn this in Mod 8, the last module of HSC Chem
The description above is correct, but I guess it doesn't answer your question as to where the red colour comes from. I.e. why is it not blue or green
These are concepts way past the HSC (and your teacher probably doesn't even want you to know it this detailed) but anyways I will try explain it in a simplified way
So first off Iron thiocyanate is what we call a metal complex. These types of compounds are made of two things:
- A transition metal ion
- Something we call a ligand (basically the thiocyanate in this case). Ligands are molecules that are able to donate an electron pair to the transition metal ion
Transition metals as you probably learnt in year 11 are things that have d orbitals. So when we write their electron configurations it always ends with something like 3d5, 3d6 etc. There are 5 different sub shells in the d-orbitals as shown below

When these ligand molecules approach a transition metal, they cause these 5 orbitals to split into 2 orbitals that move higher in energy and 3 orbitals which drop lower in energy based on if the ligand can approach them easier (sometimes 2 will go lower and 3 higher but just ignore this and consider that generalisation I said above)
This splitting of the orbitals is shown in the diagram below:

Now you can see that there is an energy gap between the 3 lower orbitals and the 2 higher orbitals. This energy gap is really important (to the colour seen). How big it is (which is affected by the type of metal and its charge and the ligands) determines the type of light that can cause an electron from the lower orbitals to jump to the higher energy orbitals
The thing about metal complexes is that the energy gap shown above usually corresponds to visible light (so the colours we can see), which is why a lot of the transition metals like iron, copper and nickel have coloured solutions
The colour red will be related to a certain energy gap, similarly to how the colour green will be related to a certain size energy gap. If that gap changed size then the colour of the iron thiocyanate would also change
The theory I have outlined above is something called Crystal Field Splitting Theory that if you do uni chemistry you will learn about in first year
These are concepts way past the HSC (and your teacher probably doesn't even want you to know it this detailed) but anyways I will try explain it in a simplified way
So first off Iron thiocyanate is what we call a metal complex. These types of compounds are made of two things:
- A transition metal ion
- Something we call a ligand (basically the thiocyanate in this case). Ligands are molecules that are able to donate an electron pair to the transition metal ion
Transition metals as you probably learnt in year 11 are things that have d orbitals. So when we write their electron configurations it always ends with something like 3d5, 3d6 etc. There are 5 different sub shells in the d-orbitals as shown below

When these ligand molecules approach a transition metal, they cause these 5 orbitals to split into 2 orbitals that move higher in energy and 3 orbitals which drop lower in energy based on if the ligand can approach them easier (sometimes 2 will go lower and 3 higher but just ignore this and consider that generalisation I said above)
This splitting of the orbitals is shown in the diagram below:

Now you can see that there is an energy gap between the 3 lower orbitals and the 2 higher orbitals. This energy gap is really important (to the colour seen). How big it is (which is affected by the type of metal and its charge and the ligands) determines the type of light that can cause an electron from the lower orbitals to jump to the higher energy orbitals
The thing about metal complexes is that the energy gap shown above usually corresponds to visible light (so the colours we can see), which is why a lot of the transition metals like iron, copper and nickel have coloured solutions
The colour red will be related to a certain energy gap, similarly to how the colour green will be related to a certain size energy gap. If that gap changed size then the colour of the iron thiocyanate would also change
The theory I have outlined above is something called Crystal Field Splitting Theory that if you do uni chemistry you will learn about in first year
This has got me interested. I can't find reliable data on the identity of the complex I.e tetrahedral or octahedral which is strange for such a common complex; nevertheless the extinction coefficient of around 4000 is very high for an d-d transition. I suspect it might be a charge transfer band, metal to ligand, but I can't be sure. If anyone has any more information, I would be very interested to hear it.
Isomorph (and hopefully others who are interested), here's a long answer to a simple question, presented because I think it's an interesting example from the History of Chemistry.
The equilibrium involving Fe3+ and SCN− has been studied for a long time. Kevin de Berg, an Adjunct Associate Professor at Avondale College near Newcastle, wrote a book on it that was published last year in the Springer's History of Chemistry series. It's Introduction includes a table listing dozens of papers on the topic of this reaction and the species responsible for the colour.
Bibliography
Bent, H. E.; French, C. L. (1941). The Structure of Ferric Thiocyanate and its Dissociation in Aqueous Solution. Journal of the American Chemical Society, 63(2):568-572. https://dx.doi.org/10.1021/ja01847a059
de Berg, Kevin C. (2019). The Iron(III) Thiocyanate Reaction: Research History and Role in Chemical Analysis. Springer International Publishing. ISBN 978-3-030-27315-6 (book) 978-3-030-27316-3 (ebook) https://dx.doi/org/10-1007/978-3-030-27316-3 (DOI link provides previews of some parts of the book plus references) https://books.google.com.au/books?id=tCy-DwAAQBAJ (Google Books link provides much of the Introduction).
Greenwood, Norman N.; Earnshaw, Alan. (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
Gladstone, John Hall. (1855). On Circumstances Modifying the Action of Chemical Affinity. Philosophical Transactions of the Royal Society, 145:179-223. https://www.jstor.org/stable/108516
Lewin, Seymour A.; Wagner, Roselin Seider. (1953). The Nature of Iron(III) Thiocyanate in Solution. Journal of Chemical Education, 30(9):445-449. https://dx.doi.org/10.1021/ed030p445
Schlesinger, H. I.; van Valkenburgh, H. B. (1931). The Structure of Ferric Thiocyanate and the Thiocyanate Test for Iron. Journal of the American Chemical Society, 53(4):1212-1216. https://dx.doi.org/10.1021/ja01355a003
The equilibrium involving Fe3+ and SCN− has been studied for a long time. Kevin de Berg, an Adjunct Associate Professor at Avondale College near Newcastle, wrote a book on it that was published last year in the Springer's History of Chemistry series. It's Introduction includes a table listing dozens of papers on the topic of this reaction and the species responsible for the colour.
- In 1826, Berzelius reported the intense red colour formed when thiocyanic acid (HSCN) is mixed with some iron compounds.
- As de Berg explains early in the introduction, there was much chemistry that was known prior to 1800 but that was not well characterised. Dalton's atomic theory appeared at the start of the 19th century, and Berzelius was an important adopter of it. He built on the work of Lavoisier in developing the symbolic representation of chemistry that we know today (though he used superscripts rather than subscripts, thus representing water as H2O rather than H2O) and his work included determining the fomulae of thousands of compounds, formulating the Law of Definite Proportions, and identification or isolation of several new elements. It is thus logical for de Berg to start the history of the systematic research on the iron(III) / thiocyanate system with Berzelius.
- Gladstone published a characterisation of the reaction in 1855. The symbolism is somewhat unfamiliar from a modern perspective, but M is a metal, Rd is a salt radical (what we would call an anion), and S2Cy is a sulphocyanide, an older name for thiocyanate. The report is under the heading "Ferric Sulphocyanide," confirming that it relates to what we know as iron in the +3 oxidation state. He reported that:
Fe2 Rd3 + 3M, S2Cy = Fe2, 3S2Cy + 3M Rd
- Gladstone's report is pointing to the product as the neutral species iron(III) thiocyanate, Fe(SCN)3, formed in what is often (and unhelpfully, IMO) termed a double displacement reaction.
- Following Gladstone's work, the consensus appears to have been that the colour was due to Fe(SCN)3 or Fe(CNS)3, and this prevailed into the 20th century. The difference in formula may indicate some appreciation for what is now understood about the thiocyanate ion, that it is capable of forming bonds through the S or N atoms, in what is now termed linkage isomerism. That is, a compound with a C6H5 (phenyl) group covalently bound to this chemical moiety may be phenyl thiocyanate, C6H5−S−C≡N, or phenyl isothiocyanate, C6H5−N=C=S.
- In 1931, a paper in the Journal of the American Chemical Society reported that the colouration was actually due to an octahedral complex, [Fe(SCN)6]3− and that the neutral form responsible for the colour in ether was an iron(III) salt of this complex, Fe[Fe(SCN)6].
- Subsequent papers included discussion of other species, including FeSCN2+, FeSCN+, [Fe(SCN)2]+, and [Fe(SCN)4]−. A paper by Bent and French in 1941 reported evidence of the presence of FeSCN2+ and a lack of evidence to support the presence of either Fe(SCN)3 or [Fe(SCN)6]3- in aqueous solution.
- Subsequent work confirms the colouration as due to FeSCN2+, though the formation of [Fe(SCN)2]+ is confirmed to contribute to the colour at higher concentrations. Fading occurs in acidic media due to the reduction of iron(III) to iron(II) associated with the oxidation of SCN− to (SCN)2, which then undergoes hydrolysis. Species like Fe(SCN)3 and Fe[Fe(SCN)6] confirmed in organic media but not in water. The research findings up to 1953 are well summarised by Lewin and Wagner.
- Mechanistic work published in 1958 confirmed that FeSCN2+ is actually an octahedral pentaaqua complex, [Fe(H2O)5SCN]2+ and its mechanism of formation is suggested to involve a three-step process of related equilibria:
[Fe(H2O)6]3+ ⇌ [Fe(H2O)5OH]2+ + H+
[Fe(H2O)5OH]2+ + SCN− ⇌ [Fe(H2O)4(OH)(SCN)]+ + H2O
[Fe(H2O)4(OH)(SCN)]+ + H+ ⇌ [Fe(H2O)5SCN]2+
[Fe(H2O)5OH]2+ + SCN− ⇌ [Fe(H2O)4(OH)(SCN)]+ + H2O
[Fe(H2O)4(OH)(SCN)]+ + H+ ⇌ [Fe(H2O)5SCN]2+
- Wikipedia also presents the specie FeSCN2+ as an octahedral pentaaqua complex, [Fe(H2O)5SCN]2+, referencing it to page 1090 of Greenwood and Earnshaw's textbook. It would be helpful for someone to check on this as the WP page also attributes the complex as being bound through the N atom, in the style of an isothiocyanate, and thus being the pentaaqua(thiocyanato-N)iron(III) ion, [Fe(H2O)5NCS]2+. See https://en.wikipedia.org/wiki/Thiocyanate#Test_for_iron(III)_and_cobalt(II)
Bibliography
Bent, H. E.; French, C. L. (1941). The Structure of Ferric Thiocyanate and its Dissociation in Aqueous Solution. Journal of the American Chemical Society, 63(2):568-572. https://dx.doi.org/10.1021/ja01847a059
de Berg, Kevin C. (2019). The Iron(III) Thiocyanate Reaction: Research History and Role in Chemical Analysis. Springer International Publishing. ISBN 978-3-030-27315-6 (book) 978-3-030-27316-3 (ebook) https://dx.doi/org/10-1007/978-3-030-27316-3 (DOI link provides previews of some parts of the book plus references) https://books.google.com.au/books?id=tCy-DwAAQBAJ (Google Books link provides much of the Introduction).
Greenwood, Norman N.; Earnshaw, Alan. (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
Gladstone, John Hall. (1855). On Circumstances Modifying the Action of Chemical Affinity. Philosophical Transactions of the Royal Society, 145:179-223. https://www.jstor.org/stable/108516
Lewin, Seymour A.; Wagner, Roselin Seider. (1953). The Nature of Iron(III) Thiocyanate in Solution. Journal of Chemical Education, 30(9):445-449. https://dx.doi.org/10.1021/ed030p445
Schlesinger, H. I.; van Valkenburgh, H. B. (1931). The Structure of Ferric Thiocyanate and the Thiocyanate Test for Iron. Journal of the American Chemical Society, 53(4):1212-1216. https://dx.doi.org/10.1021/ja01355a003
Jazz519 offers an excellent summary of the reasons for the colours. I would like to add that:
- The narrow difference in energy of d orbitals allowing for transitions with energies that correspond to visible colour changes is actually included in the high school course for students in the current IB programme (especially in the higher level) and it has appeared in older HSC syllabi under the Chemistry of Art.
- The reasons for the splitting of orbitals into two groups is intuitively clear if one considers the geometry of the orbitals themselves and the complex. An octahedral complex has ligands whose positions relative to the metal centre correspond to the positive and negative directions of the x, y, and z coordinate axes. There are three d orbitals which point between these axes (dxy, dyz, and dzx) and two d orbitals that point directly along one or more coordinate axes (dx2-y2 and dz2). Qualitatively, you can think of the latter two orbitals as having higher energy because their electrons are oriented towards the electron density of the ligand, which is destablising.
- On Isomorph's question, the Chemistry textbook for IB students shows the formula as the pentaaqua form on page 110, where there is also a discussion of this phenomenon (pages 110-114). https://www.academia.edu/34765684/C...r_the_IB_Diploma_Chemistry_for_the_IB_Diploma
- The energy gaps between orbitals leading to coloured lines is the basis for atomic emission spectroscopy and atomic absorption spectroscopy, covered in both the IB and HSC courses. This is not a phenomenon restricted only to d orbitals, though they are the source of much colour in chemistry, and especially in transition metal chemistry.
- Jazz's illustration of hexacyanoferrate(II) as a low spin with six electrons in d orbitals is correct, but IB students should note that there are also high spin complexes where all the d orbitals fill before any pairing occurs, and this will lead to a difference in magnetism. A low spin iron(II) complex will have no unpaired electrons and will be diamagnetic. A high spin iron(II) complex will have four unpaired electrons (plus a pair in one of the three degenerate orbitals of lower energy) and so be paramagnetic.
Wow, thank you for presenting the interesting history of the complex!
Regarding the colours, I believe that it is much more complex than appears at first sight. For example, one might pose the question of, why is the complex [Fe(H2O)6]3+ much less intensely coloured than [Fe(H2O)5(NCS)]2+, given that they are both high spin d5 complexes? Additionally, why are the complexes differently coloured, given 5 of the 6 ligands are identical in both complexes, and the 6th ligands are right next to each other on the spectrochemical series (see below) i.e of similar splitting strengths, hence we would expect a similar splitting energy?

We can check the absorption spectra to confirm that the absorptions occur at subtantially different wavelengths, which is incongruent with both transitions being d-d.
Iron thiocyanate (absorbs 450-500nm, appears red):

Iron Nitrate (Absorbs at a lower wavelength, appears yellow)
In the graph below, the phosphoric acid converts the ionised iron complex back (i.e [Fe(OH)(H2O)5]2+ back into [Fe(H2O)6]3+, so we are interested in the dotted line.

We can rationalise these findings, by supposing that while the iron hexaaqua complex is a d-d transition between the two orbitals, as Jazz519 noted, the iron thiocyanate compelex exhibits a completely different transition.
We can construct a orbital energy diagram that depicts the various energy levels of the entire iron thiocyanate complex, and it would look something like below. The d-d splitting that is mentioned can be seen in the familiar 3-2 splitting, here named t2g* and eg*. Orbitals would be fully filled up to the t2g set, and half filled for the t2g* and eg* sets. The transition we are interested in is t2g -> t2g*. As the t2g orbital mainly comes from the p orbital set on the ligand (the right column), and the t2g* mainly comes from the metal, we can term such a transition "ligand to metal". Also note that the p-orbitals, although reflected as the t2g set, can also be thought of a lone pair or pi bond on the ligand, that transfers its electrons to the metal i.e "reduces the metal".
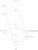
Finally, we should rationalise the intensity of the transition. The d-orbital splitting diagram of high spin d5 complexes have each orbital half filled, electrons with the same spin. There is a selection rule, that transitions cannot occur (are weaker) when the electron is required to change spin going from one orbital to the other. Clearly, this is required for these complexes (due to the principle that in an orbital, the two electrons are of opposite spins), hence the d-d transition is weak (as expected for the hexaaqua complex). However, you can notice that in the ligand to metal transition of the thiocyanate complex, there is always an electron in the filled t2g set that can transition to the t2g* set without changing spin i.e it is allowed, hence the intensity is very strong.
For reference, here is a filled splitting diagram for high spin d5
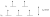
Reference to the LMCT: Page 291 of
Electronic Absorption Spectroscopy and Related Techniques
By D. N. Sathyanarayana
This book is also very good for understanding electronic transitions and colours of various complexes.
Regarding the colours, I believe that it is much more complex than appears at first sight. For example, one might pose the question of, why is the complex [Fe(H2O)6]3+ much less intensely coloured than [Fe(H2O)5(NCS)]2+, given that they are both high spin d5 complexes? Additionally, why are the complexes differently coloured, given 5 of the 6 ligands are identical in both complexes, and the 6th ligands are right next to each other on the spectrochemical series (see below) i.e of similar splitting strengths, hence we would expect a similar splitting energy?

We can check the absorption spectra to confirm that the absorptions occur at subtantially different wavelengths, which is incongruent with both transitions being d-d.
Iron thiocyanate (absorbs 450-500nm, appears red):

Iron Nitrate (Absorbs at a lower wavelength, appears yellow)
In the graph below, the phosphoric acid converts the ionised iron complex back (i.e [Fe(OH)(H2O)5]2+ back into [Fe(H2O)6]3+, so we are interested in the dotted line.

We can rationalise these findings, by supposing that while the iron hexaaqua complex is a d-d transition between the two orbitals, as Jazz519 noted, the iron thiocyanate compelex exhibits a completely different transition.
We can construct a orbital energy diagram that depicts the various energy levels of the entire iron thiocyanate complex, and it would look something like below. The d-d splitting that is mentioned can be seen in the familiar 3-2 splitting, here named t2g* and eg*. Orbitals would be fully filled up to the t2g set, and half filled for the t2g* and eg* sets. The transition we are interested in is t2g -> t2g*. As the t2g orbital mainly comes from the p orbital set on the ligand (the right column), and the t2g* mainly comes from the metal, we can term such a transition "ligand to metal". Also note that the p-orbitals, although reflected as the t2g set, can also be thought of a lone pair or pi bond on the ligand, that transfers its electrons to the metal i.e "reduces the metal".
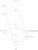
Finally, we should rationalise the intensity of the transition. The d-orbital splitting diagram of high spin d5 complexes have each orbital half filled, electrons with the same spin. There is a selection rule, that transitions cannot occur (are weaker) when the electron is required to change spin going from one orbital to the other. Clearly, this is required for these complexes (due to the principle that in an orbital, the two electrons are of opposite spins), hence the d-d transition is weak (as expected for the hexaaqua complex). However, you can notice that in the ligand to metal transition of the thiocyanate complex, there is always an electron in the filled t2g set that can transition to the t2g* set without changing spin i.e it is allowed, hence the intensity is very strong.
For reference, here is a filled splitting diagram for high spin d5
Reference to the LMCT: Page 291 of
Electronic Absorption Spectroscopy and Related Techniques
By D. N. Sathyanarayana
This book is also very good for understanding electronic transitions and colours of various complexes.
Attachments
-
368.1 KB Views: 1
-
119.8 KB Views: 2
I should mention for those interested - that the charge transfer does not have to occur on the same species! For example, the triphenylphosphine - iodine charge transfer complex is one, where an electron transfers for the triphenylphosphine to the iodine, in essence forming [PPh3I]+ I-
While the complex is forming, the energy difference of the charge transfer is provided by light of a specific wavelength, resulting in spectacular colours (see below). Once the charge transfer complex is formed, the colour disappears (3rd test tube). The fourth test tube is darkly coloured due to triodide ion I3- . Note that in this case, charge transfer complex is permanent - but it might not be, e.g in our transition metal complexes, where the electron falls back down and can be re-excited!

If it helps you understand the previous ligand-> metal transition, you can also think of the complex with the thiocyanate "unbound" from the iron, i.e [Fe(H2O)5]3+ SCN- and think of the transition from a pi/p electron of the thiocyanate to the orbitals that are triply degenerate (three on the same energy level) on the iron, labelled as t2g* on the huge diagram and t2g on the simplified diagram
While the complex is forming, the energy difference of the charge transfer is provided by light of a specific wavelength, resulting in spectacular colours (see below). Once the charge transfer complex is formed, the colour disappears (3rd test tube). The fourth test tube is darkly coloured due to triodide ion I3- . Note that in this case, charge transfer complex is permanent - but it might not be, e.g in our transition metal complexes, where the electron falls back down and can be re-excited!

If it helps you understand the previous ligand-> metal transition, you can also think of the complex with the thiocyanate "unbound" from the iron, i.e [Fe(H2O)5]3+ SCN- and think of the transition from a pi/p electron of the thiocyanate to the orbitals that are triply degenerate (three on the same energy level) on the iron, labelled as t2g* on the huge diagram and t2g on the simplified diagram
Attachments
-
309.8 KB Views: 0
Last edited:
I certainly can't disagree that such a high extinction coefficient is unusual for a d-d transition. The greater detail in looking at molecular orbitals etc is definitely well beyond HSC or IB chemistry.This has got me interested. I can't find reliable data on the identity of the complex I.e tetrahedral or octahedral which is strange for such a common complex; nevertheless the extinction coefficient of around 4000 is very high for an d-d transition. I suspect it might be a charge transfer band, metal to ligand, but I can't be sure. If anyone has any more information, I would be very interested to hear it.
Yeah I looked into it mostly out of interest and curiosity especially given it was such a common complex ion.
Although, the IB syllabus does mention "Explanation of the effect of the... identity of the ligand on the colour of transition metal ion complexes.", and on p114 of the book you linked, it mentions pi-acceptor and pi-donor complexes, so it still could be useful to at least know these transitions in general terms, e.g ligand to metal, even if we are not expected to produce MO diagrams.
Although, the IB syllabus does mention "Explanation of the effect of the... identity of the ligand on the colour of transition metal ion complexes.", and on p114 of the book you linked, it mentions pi-acceptor and pi-donor complexes, so it still could be useful to at least know these transitions in general terms, e.g ligand to metal, even if we are not expected to produce MO diagrams.